In this unit we explore the connections between molecular polarity and the attractive forces between molecules. These subtle yet powerful interactions dictate much of the physical and chemical properties substances have on the macroscopic scale.
Required Reading
- Chemistry 10e, Zumdahl:
- Chapter 10 (except section 10.8)
- Chapter 5
Friday, 3 November
In class
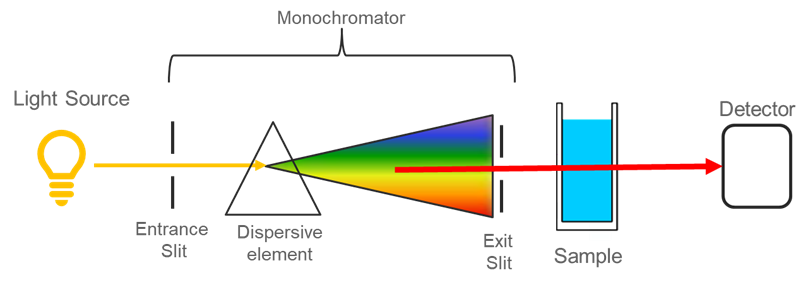
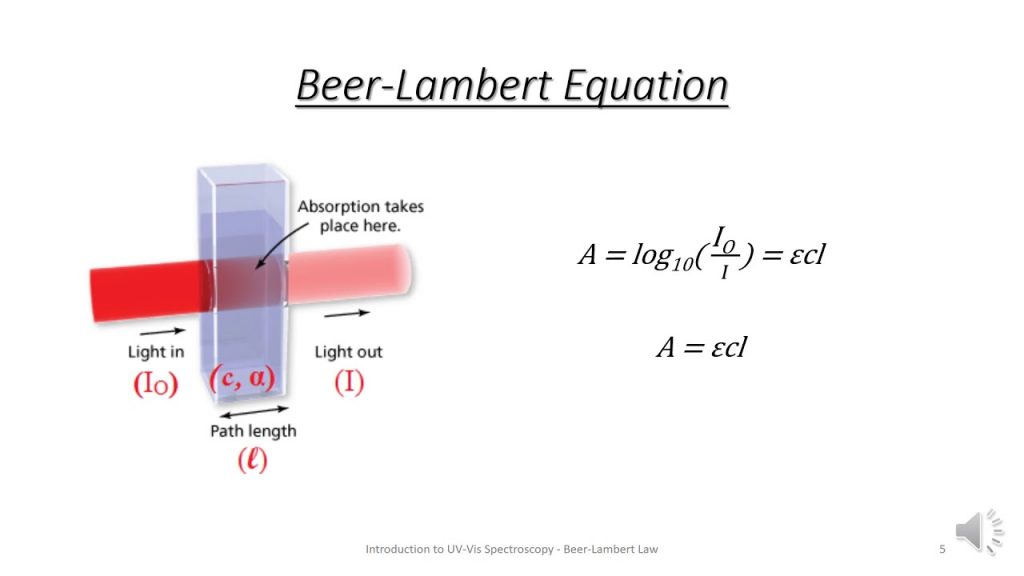
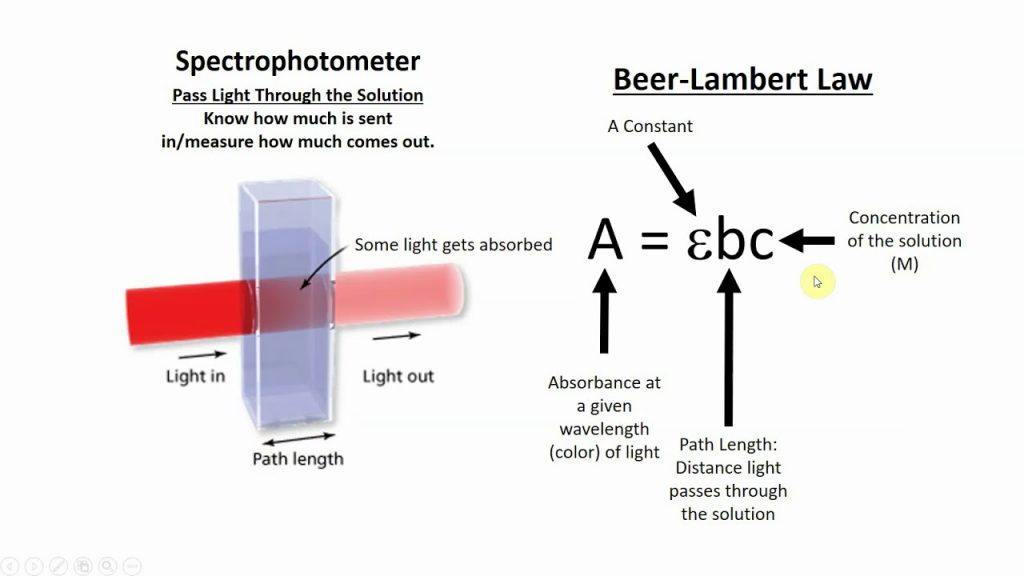
- Short-form assessment: Beer-Lambert law and visible spectroscopy.
Thursday, 2 November
In class
- Putting it all together: the percent mass of copper in a sample of brass.
- Review session for the Unit 3 exam.
Assignments
- Review your notes from today’s discussion in preparation for a short experiment assessment tomorrow on visible spectroscopy.
- Read the introduction to Chapter 4 and section 4.1.
Wednesday, 1 November
In class
- Measure absorption of brass sample.
- Determine the molarity of copper(II) ions in the brass sample and then calculate the mass of copper present and the percentage copper in brass.
Assignments
- Finish the Unit 3 Progress Check by tomorrow to prepare for our review session.
Tuesday, 31 October
In class
- How can we determine the mass percent of copper in brass?
- Finish creating calibration curve and discuss.
- The reaction of brass with nitric acid.
- Measuring the absorption of the brass sample.
Assignments
- Continue work on the Unit 3 Progress Check.

Monday, 30 October
In class
- Equipment talk: the Vernier SpectroVis Plus spectrophotometer.
- Determining the optimal wavelength to measure our copper(II) solution.
- Creating a calibration curve.
Assignments
- Dress for laboratory work tomorrow.
- Begin work on the Unit 3 Progress Check.
Friday, 27 October
In class
- Review practice quizzes.
- Practice FRQ: chromatography
- How can we determine the mass percent of copper in brass?
- Beer-Lambert Law PhET simulation
Assignments
- Dress for laboratory work on Monday.
- Complete the work for Topics 3.11 – 3.13 in APC before class Tuesday.

Wednesday, 25 October
In class
- Determination of the food dyes in M&M candies by paper chromatography

Tuesday, 24 October
In class
- Review assignment on molarity calculations.
- Practice: Molarity
- Principles of paper chromatography
Assignments
- Dress for laboratory work tomorrow.

Monday, 23 October
In class
- Warm up exercise: The Maxwell-Boltzmann distribution.
- How do scientists describe the concentration of a solution?
- Molarity
- Making a Molar Solution
- Making a NSS.
- Homeopathic medicine and the molar limit
Assignments
- Complete the Solutions and Mixtures practice quiz in APC before the start of class tomorrow.
- Watch the section 3.9 videos on Separation of Solutions and Mixtures Chromatography in APC.
Friday, 20 October
In class
- IMF short-form assessment.
- Continue work on the Maxwell-Boltzmann distribution problem set.
Assignments
- Watch the assigned APC videos for section 3.7 Solutions and Mixtures and 3.8 Representations of Solutions.
- Read section 11.1 in Chemistry 10e.
- Dress for laboratory work on Monday.
Thursday, 19 October
In class
- Whiteboard session: Deviations from the Ideal Gas Law.
- How does temperature change the speed of gas particles?
- Gas Properties PhET simulation
Assignments
- Review Topics 3.1 – 3.3 for a quiz tomorrow. I released three practice quiz questions in APC that you may find useful.

Wednesday, 18 October
In class
- Feedback on the Unit 2 exam.
Assignments
- Finish the Deviations from the Ideal Gas Law problem set before class tomorrow.
- Quiz on Friday covering sections 3.1 – 3.3.
Tuesday, 17 October
In class
- Warm up exercise: boiling points and IMFs
- Ideal gas law problem set and whiteboards.
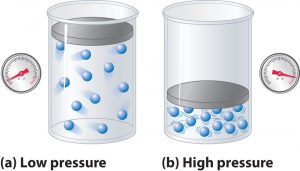
- How well does the ideal gas law predict the pressure of gases?
Assignments
- Work through Models 1 & 2 in the Deviations from the Ideal Gas Law problem set for tomorrow. Finish the problem set for Thursday.
Monday, 16 October
In class
- Whiteboards: IMF experiment.
Assignments
- Watch the APC videos for sections 3.4 and 3.5.
- Over the next few days for review, read sections 5.1 – 5.3, 5.5, and 5.6

Friday, 13 October
In class
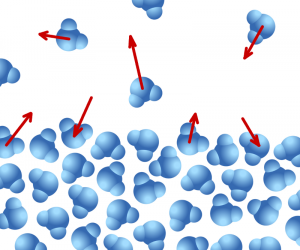
- How can we experimentally investigate the relationship between evaporation, temperature, and IMFs for a series of volatile liquids?
Assignments
- Organize your data into a table in your notebook if you have not done so already.
- Write a rough draft of your claim, evidence, and reasoning on the experiment. Your groups will present this on Monday in a whiteboard session.
Thursday, 12 October
In class
- Discuss APC practice quiz questions on solids.
- How can we relate IMFs to boiling points?
Assignments
- Dress for laboratory work tomorrow.

Wednesday, 11 October
In class
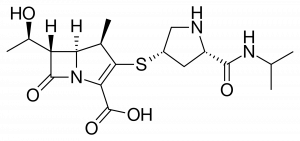
- What type of intermolecular forces are present in the molecule meropenem?
- Another look at classifying solids. Patterns with intramolecular forces and intermolecular forces.
Assignments
- Complete the Properties of Solids Quiz in APC before the start of class tomorrow.
Tuesday, 10 October
In class
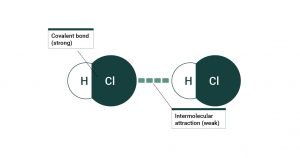
- How do chemist’s distinguish between the different types of intermolecular forces?
Friday, 6 October
- Unit 2 assessment.
Thursday, 5 October
In class
- Discuss assignment exercises.
- Is meropenem a polar or non polar molecule?
- Answer any final questions before tomorrow’s Unit 2 assessment.

Wednesday, 4 October
In class
- Observing phenomena:
- Surface tension of liquids.
- The Big Picture of intermolecular forces (IMFs).
- What makes a molecule polar?
- Molecule Polarity PhET simulation.
Assignments
- Zumdahl Chapter 8 exercise(s): 113. Then, using the simulation, state whether each species is polar or nonpolar.
