Tuesday, 10 September
In class
- Turn in empirical formula analysis questions if you didn’t do so yesterday.
- Feedback on the most recent quiz. (Worked solutions posted on the secure page.)
- Unit 1 whiteboard.
Assignments
- Read this CER exemplar paper and compare with what you and your group wrote for the stainless steel analysis experiment. In your laboratory notebook comment (sometime in the next week or so) on:
- what your group did well.
- what needs to be improved upon for the next experiment CER.
- Bring which ever device you plan to take the AP exam on this May with you tomorrow.
Monday, 9 September
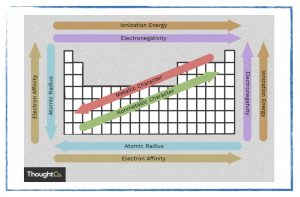
Why does the periodic table look like that?
In class
- Q&A on the electron configuration and PES APC quizzes.
- Trends in the periodic table.
Exercises
- The first unit exam is coming soon. Begin reading through Chapters 1 & 2 in the Barron’s review book.
Assignments
- Complete the APC quizzes on Periodic Trends and Valence Electrons and Ionic Compounds.
- Begin work on the Unit 1 Progress Check in APC.
Friday, 6 September
What experimental evidence do we have for the existence of atomic orbitals?
In class
- Quiz: isotopes, mass spectroscopy, and empirical formulas.
- Finish any last measurements for the magnesium oxide experiment and clean up.
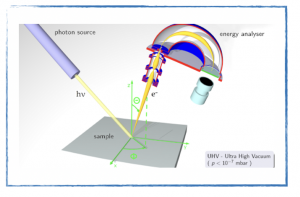
- Photoelectron spectroscopy.
Assignments
- Complete the analysis questions for the empirical formula experiment by Monday.
- Complete the Atomic Structure and Electron Configuration and Photoelectron Spectroscopy quizzes in APC before the start of class on Monday.
- Finish reading Chapter 6 by Monday. As always, test your understanding by attempting the exercises at the end of the chapter and check your answers with the student solution manual.
Thursday, 5 September
What is the basic premise of quantum mechanics as applied to electrons in atoms?
In class
- Heating of the magnesium oxide sample.
- Crash course in quantum mechanics.
Assignments
- Review for a quiz tomorrow on isotopes, mass spectroscopy, and empirical and molecular formulas.
- Upload your video to the Classroom assignment page.
Wednesday, 4 September
How can we experimentally determine the empirical formula for a magnesium oxide?
In class
- Discuss the experiment objectives and procedure.
- Form lab groups and carry out the experiment.
Exercises
- Begin reading Chapter 3: Electronic Structure and Periodic Properties of Elements. We will only go into detail on a few of the topics in this chapter but it is worth reading at least once to gain a sense of the general ideas in quantum mechanics.
Assignments
- Submit your groups’ 90 second video before the end of the day tomorrow to google classroom.

Tuesday, 3 September
How can combustion analysis determine the empirical formula for an organic compound?
In class
- Q&A on empirical and molecular formulas.
- Combustion analysis.
Exercises
- Problems 7 – 18 in the Exercises section of Chapter 6 will provide you with practice on determining empirical and molecular formulas.
Assignments
- Dress for laboratory work tomorrow.
- Read the introduction and procedure for the experiment Determining the Empirical Formula of a Magnesium Oxide before class tomorrow. Make note of any questions that you have.
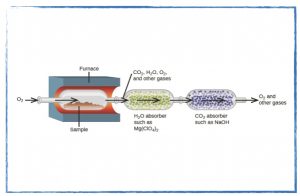
Friday, 30 August
How can the chemical formula of a compound be determined?
In class
- Finish and discuss the mass spectroscopy problem set.
- Calculating the percent composition of a pure substance.
- The empirical formula of a compound.
Assignments
- Read the introduction and sections 1 and 2 of Chapter 6.
- I’m also releasing quizzes in APC for sections 1.3 and 1.4 to have finished before class on Wednesday.

Thursday, 29 August
In class
- A short quiz on the review problem set.
Assignments
- Please print one copy of your experiment CER to turn in at the start of class tomorrow.
Wednesday, 28 August
How do we know that isotopes exist?
In class
- Discuss particle diagrams.
- The subatomic structure of atoms.
- Isotopes and mass spectroscopy
- POGIL paper roles.
- Mass spectroscopy problem set.
Assignments
- Complete the quiz Mass Spectra of Elements in APC before the start of class on Friday.
- Looking over your work and notes from the review problem set in preparation for the quiz tomorrow.
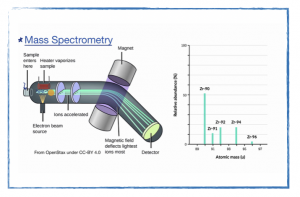
Tuesday, 27 August
What model(s) can best represent the states of matter in chemical reactions?
In class
- Moles and molar mass.
- Drawing particle diagrams.
Exercises
- I would suggest that you should be reading section 2.3 at this point in the week. We will begin discussing atomic structure tomorrow.
Assignments
- Update your laboratory notebook to have the particle diagram for the zinc plated steel experiment as before, during, and after.
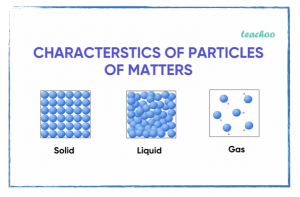
Monday, 26 August
How many layers of zinc are on a typical sample of stainless steel?
In class
- Group discussion of the experiment calculations.
- How to write a CER paper.
- Q&A on the review problem set.
Exercises
- Begin reading chapter 2, Atoms, Molecules, and Ions.
Assignments
- Log in to AP Classroom (APC) and watch the daily videos for section 1.1.
- Complete the assigned Moles and Molar Mass quiz on APC.

Friday, 23 August
How does one determine how many significant figures an instrument measures to?
In class
- Procedure, safety, and clean up discussion.
- Carry out the data and collect data.
- Begin analysis work.
Exercises:
- Give Chapter 1 a quick read, with special attention to sections 1.4 – 1.6. This should help you with any questions you may have with the review problem set.
Assignments:
- Finish the review problem set before class on Monday. A quiz on this material will be given next week.
- In your laboratory notebook using your group’s data:
- calculate the number of zinc atoms.
- calculate the number of layers of zinc atoms.
- sketch particle diagrams for the reaction between zinc and hydrochloric acid at the points, before, during, and after reaction completion.
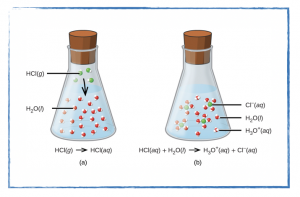
Thursday, 22 August
What information does a balanced chemical equation provide?
In class:
- Welcome and introductions.
- Setting up the course resources
- A stoichiometry review problem.
- Your first experiment: Quantitative Analysis of Zinc Atoms in Zinc-Plated Steel.
Assignments:
- Bring all required materials to class tomorrow.
- Begin work on the Chemistry Fundamentals Review Problem Set.
- Dress for laboratory work tomorrow.
- Watch the following videos on laboratory techniques you will use tomorrow:


