Thursday, 24 October
In class
- Unit 3 whiteboard problem set. pdf
Assignments
- Continue your review and revisions for the Unit 3 exam on Monday.
- Check that your laboratory notebook is updated and ready to turn in Monday as well.
Wednesday, 23 October
In class
- Happy Mole Day!
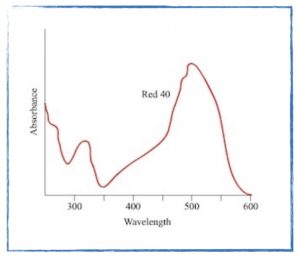
- Analyzing the data from the spectroscopy experiment.
- Sample Data Set (if your data are a bit funky!)
Assignments
- Review and bring your questions to class tomorrow for a Q&A session.
Tuesday, 22 October
In class
- Experimental determination of the concentration of blue dye in Glacial Freeze Gatorade
- Vernier Spectral Analysis software download
Assignments
- Create the plots for your experimental data if you didn’t get to in class today.
- Begin work on the Unit 3 Progress Checks in APC.
Monday, 21 October
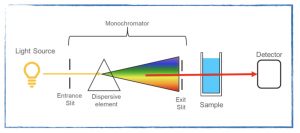
How is electromagnetic radiation used to analyze matter?
In class
- Welcome and a brief statement on political speech.
- Happy National Chemistry Week!
- Warm up Exercise: Calculating the wavelength of electromagnetic radiation.
- Spectroscopy problem set.
- Beer’s Law PhET simulation
Assignments
- Dress for laboratory work tomorrow.
- Watch the following videos on the techniques needed for the food dye analysis experiment tomorrow.
- Looking ahead, the Unit 3 exam will be given next Monday.
Friday, 18 October
How can we use an understanding of intermolecular forces to separate mixtures?
In class
- Turned in the Molar Volume of Hydrogen Gas experiment paper.
- Intermolecular forces quiz.
- Finished the Solubility and the Solution Process problem set.
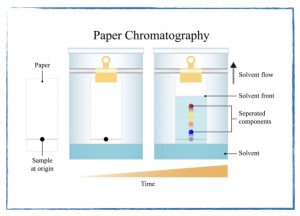
- How chemists separate mixtures.
- Problem Set: Chromatography.
- Our Favorite Songs
Assignments
- Finish work on the Chromatography problem set if you didn’t do so during class time.
- Read section 3.1, paying close attention to the relationship between frequency, wavelength, and energy of particular colors of light.
- Watch the video Every Other Video About Color is Wrong. After watching the video you should be able to:
- State and explain the definition of color.
- Explain how a molecule’s structure relates to the color we perceive it as.
Thursday, 17 October
How do the types of intermolecular forces possible in a compound affect its ability to form a solution with another compound?
In class
- Whiteboard session: Solubility and the Solution Process.
Assignments
- Review for the quiz tomorrow on IMFs.
- Read the following sections in your textbook:
- 10.2, focusing upon the discussion of adhesive and cohesive forces.
- 11.1, focusing on the process of solution formation.
- 11.3, focusing on the what properties of a compound make it able to dissolve in another compound.
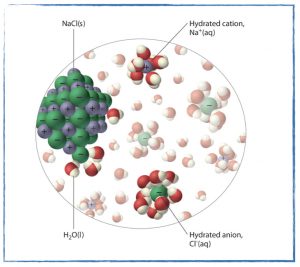
Wednesday, 16 October
How well does the ideal gas law predict the molar volume of a gas?
In class
- I collected your analysis paper for the Making a Molar Solution experiment.
- Experiment: Measuring the Molar Volume of a Sample of Hydrogen Gas.
Assignments
- Each group is to prepare a one page, typed summary of the experiment due on Friday. (Read this exemplar paper for experiment if you were unsure of all steps we took. Note that we didn’t use Equation 5 and estimated the value instead.) You only need to include the following sections:
- Data. Create a table that summarizes all of the measurements made and values researched.
- Calculations. Using the Ideal gas law, show the calculations carried out to determine the molar volume of hydrogen gas at STP.
- Percent Error. Calculate the percent error between your molar volume at STP with the accepted value.
- Friday you will take a quiz on intermolecular forces. Review the following:
- Section 10.1 in your textbook,
- The APC videos and the two relevant quizzes (Intermolecular and Interparticle Forces and Properties of Solids.)
- Barron’s: p. 254: 1 – 6, 9.
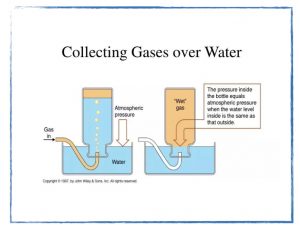
Tuesday, 15 October
What does molarity (M) tell us about the composition of a solution?
In class
- Preparing a normal saline solution.
Assignments
- Individually (but feel free to collaborate with your group) complete the analysis questions for Making a Molar Solution to turn in at the start of class tomorrow.
- Upload your group’s how-to video to Classroom before class tomorrow.
- Tomorrow you will be back in the laboratory to measure the molar volume of a sample of hydrogen gas. Please watch the video before coming to class on the technique required to carry out the experiment.

Friday, 11 October
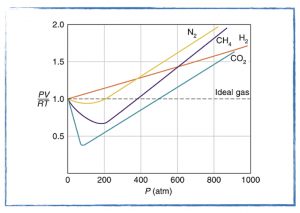
Does the Ideal Gas Law accurately predict the pressure of a gas?
In class
- Warm up exercise: Using the ideal gas law, calculate the pressure of 4.000 moles of nitrogen gas in a 10.000 L container at 22.00 C.
- Q&A on the Maxwell-Boltzmann Distributions problem set.
- Discussion of yesterday’s challenge question.
- Deviations from the Ideal Gas Law.
- Feedback on the Unit 2 exam.
Exercises
- Read sections 8.6 (deviations from ideal gas law behavior) and 6.3 (calculating concentration of solutions using molarity).
Assignments
- On Tuesday you and your working group will be asked to make a saline solution of a given molarity. Watch the video tutorial on the proper technique for making these types of solutions.
Thursday, 10 October
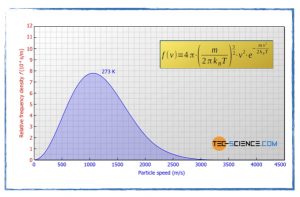
How does the temperature change the speed of particles in the gas phase?
In class
- The Maxwell-Boltzmann distribution.
- Gas Properties PhET simulation
Exercises
- Section 10.1 of your text covers the key ideas for intermolecular forces that are used to explain the properties of substances in the solid and liquid phase. Exercises 1 – 21 are good if you need more practice.
- Chapter 8 discusses gases. This should be review for all of you from first year chemistry. Look through sections 8.1, 8.2, and 8.5 for a reminder of the gas laws. Section 8.6 is the new topic that we will cover in detail tomorrow.
Wednesday, 9 October
In class
- Unit 2 exam.
Tuesday, 8 October
In class
- Problem set: Identifying Intermolecular Forces
- Practice exercises:
- Intermolecular and Interparticle Forces (APC)
Assignments
- Complete your note sheet and review for the Unit 2 exam tomorrow.
Monday, 7 October
In class
- Catch up.
- Unit 2 exam review.
- Review of the Unit 3 topics covered last week.
Assignments
- Review for the Unit 2 exam on Wednesday.
Monday, 30 September
{Text}
In class

