Friday, 27 September
- Quiz: Lewis structures and resonance.
- Time to catch up on any reading or exercises from Unit 2.
Exercises
- The Unit 2 Progress Check in APC is now open for you to begin your review and revision for the exam that will be given on Wednesday, 9 October.
Thursday, 26 September
How does one determine if a bond between two atoms is ionic, covalent, or metallic?
In class
- Valence bond theory.
- Visualizing hybrid orbitals. (Hybrid orbitals explained)
- Memorize This!
- Types of Bonds problem set.
Assignments
- Review for a short quiz tomorrow on Lewis structures and resonance.
Wednesday, 25 September
Why does VSEPR fail as an explanation for molecular shape when we consider quantum mechanics?
In class
- Valance bond theory.
- Practice quizzes on the first half of Unit 2 topics.
Assignments
- Read sections 5.1 and 5.2. A fair warning: this material is rather dense and can be challenging the first time through. Your aim is to read it and take away a general idea of the theories being presented.
Tuesday, 24 September
How can we predict the three dimensional shapes of molecules?
In class
- Q&A on any of the formal charge exercises.
- Valence Shell Electron Pair Repulsion (VSEPR) theory.
- Molecule Shapes PhET simulation.
- Chapter 4 Exercises: 76, 77, 81 – 83, 85.
Exercises
- By the end of today you should have finished reading Chapter 4.
- Read the Chapter 5 introduction and section 5.1.
Monday, 23 September,
How does one decide between equally valid Lewis structures?
In class
- Using formal charge to evaluate Lewis structures.
- Whiteboard problem set, Chapter 4: 66 – 70.
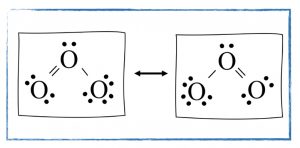
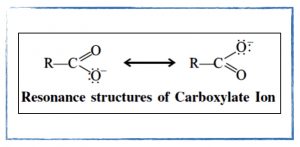
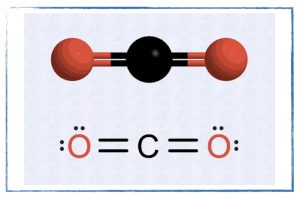
Friday, 20 September
What if one Lewis structure isn’t enough for a molecule?
In class
- Assignment check in: Chapter 4, 46.
- Feedback and discussion of the Unit 1 exam.
- Exam retake policy
- Resonance structures.
Exercises
- Chapter 4 Exercises: 55, 58, and 60.
Assignments
- Read section 4.5 by Monday and section 4.6 by Tuesday.
Thursday, 19 September
What information do Lewis structures give us about a molecule?
In class
- A more general method for drawing Lewis structures of molecules.
- Problem Set, Chapter 4: 34 (b-e), 37 (a,b), 39, and 46.
Exercises
- I’ve assigned the topic question quizzes for sections 2.1 and 2.2 – 2.4. See how well those go for you in the next couple of days.
Assignments
- Homework Check In tomorrow! I’ll will walk around to see that you attempted 46 in the above problem set at the start of class tomorrow.
Wednesday, 18 September
Why is brass considered an alloy?
In class
- Alchemy with pennies.
Exercises
- Begin reading the rest of Chapter 4 understanding that we will spend time in class discussing these topics. Just having the terms and ideas in your head will help.
Assignments
- Answer the Analysis questions 1 – 4 for the Penny Alchemy experiment in your notebook.

Tuesday, 17 September
How do metal atoms bond together?
In class
- The sea of electron model of metals.
- Observing the metal gallium.
- Alloys problem set
Exercises
- You should be through section 4.5 by tomorrow.
Assignments
- Complete the Alloys problem set and check your work with the answers on the secure page.
- Dress for laboratory work tomorrow.
- Bring three of the cleanest pennies you can find.

Monday, 16 September
In class
- Unit 1 exam
Friday, 13 September
In class
- Q&A session for any Unit 1 topics.
Assignments
- Review for the Unit 1 exam on Monday.
- Bring a fully charged device with the lockdown browser installed.

Thursday, 12 September
How do chemists classify the major types of chemical bonds?
In class
- New seats for Unit 2, if you want them.
- Discuss any questions from the Atomic Interactions problem set.
- The three main types of chemical bonds.
- The formation of ionic compounds and modeling lattice structures with Play Doh.
Assignments
- Come to class tomorrow with any last questions on the topics in Unit 1 before your unit exam on Monday.
Wednesday, 11 September
How do chemical bonds represent a stable arrangement of atoms?
In class
- Setting up the lockdown browser in APC.
- Exploring the energy interactions of atoms.
- Atomic Interactions PhET simulation
- Guided inquiry questions pdf
Assignments
- Complete the Lockdown Practice Quiz so I know that your device is up and running.
- Finish the Atomic Interactions guided inquiry questions if you did not do so during class today.
- Begin reading Chapter 4 in your textbook.