Friday, 10 May
In class
- Peer review and discussion of the solution stoichiometry problem set.
Wednesday, 8 May
In class
- Finish and discuss the Molarity problem set.
- What is the molarity of a normal saline solution?
- Practice making the molar solution.
- Film an instructional video making your solution and upload the video to Classroom.
- Answer the lead ion precipitation reaction from last class.
Assignments
- Complete the solution stoichiometry problem set before the start of class on Friday.

Monday, 6 May
In class
- Limiting reactant stoichiometry quiz.
- How many milliliters of 2.5 M sodium chloride solution is needed to precipitate 1.75 g of lead(II) nitrate out of solution?
- How can the concentration of a solution be expressed quantitatively?
- Molarity PhET simulation
Assignments
- Watch Making a Molar Solution and take notes on the steps involved.
- Bring your goggles to class Wednesday and be prepared to make your own molar solution using the technique outlined above.
Thursday, 2 May
In class
- Feedback on the Mass-to-Mass Stoichiometry Quiz.
- Measure the mass of calcium carbonate synthesized and finish the analysis questions.
Assignments
Tuesday, 30 April
In class
- Mass-to-mass stoichiometry quiz.
- Making chalk: The Synthesis of Calcium Carbonate.

Friday, 26 April
In class
- Mass-to-mass stoichiometry practice.
- Limiting and Excess Reactants
- Example exercises.
- Problem Set
Assignments
- Review mass-to-mass stoichiometry for a quiz next class. You are allowed a 3×5 notecard for this quiz.
Wednesday, 24 April
In class
- Practice quiz: mass-to-mass stoichiometry.
- How can we determine if there is enough reactants to form a certain amount of product?
- The S’mores analogy.
Assignments
- Begin work on the Limiting and Excess Reactants problem set for next class.

Monday, 22 April
In class
- Happy Earth Day!
- Fizzy Drink Stoichiometry

Thursday, 18 April
In class
- The Three Balloons.
- Mole Ratios problem set.
- Form working groups.
- Assign roles.
- Complete the problem set.
Assignments
- Complete sections 16.3 and 16.4 in cK-12 before the start of class on Monday.
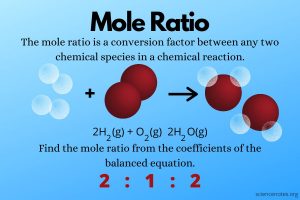
Tuesday, 16 April
In class
- Feedback on the Chemical Equations Unit Quiz.
- Guiding Question: How many grams of water will be formed if a balloon with 0.740 g of hydrogen gas is exploded in the room?
Assignments
- Complete sections 16.1 and 16.2 in cK-12 before next class.

Friday, 12 April
In class
- Discuss the Stoichiometry Unit Learning Outcomes paper.
- POGIL implementation:
- Group member roles.
- Complete the problem set.
- Whiteboard presentation
- Exit slip quiz
Assignments
- Read, complete the practice, and submit sections 14.1 – 14.3 in cK-12 before class on Tuesday.

Monday, 8 April
In class
- Chemical Reactions Unit Quiz.
- How can we count the number of atoms in a sample using a balance?
- The concept of the mole.
- Molar mass.
Assignments
- Begin and complete the Khan Academy assignments on Moles and Molar Mass by the end of the week.
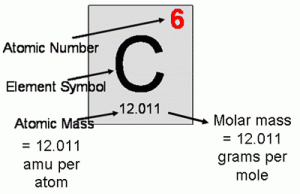
Thursday, 4 April
In class
- Reviewed how we can predict the products of single replacement reactions using the metal activity series as well as writing net ionic equations.
- How can we count the number of atoms in a sample using a balance?
Assignments
- Review for the chemical reactions unit quiz on Monday.

