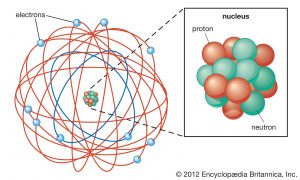
Welcome to our unit on the structure of the atom, where we embark on a fascinating exploration of the microscopic realm that forms the foundation of chemistry. We will unravel the intricacies of atoms, investigating their subatomic particles, electron configurations, and the profound role they play in shaping the physical and chemical properties of matter.
Required Readings:
- cK-12, Chapters 6 & 7
- Chemistry: Atoms First, Chapter 2 and Chapter 3, sections 3.1 – 3.4
Monday, 20 November
In class
- Unit 2 assessment.

Thursday, 16 November
In class
- Writing a technical report for the sunscreen experiment.
- Preparation for the Unit 2 assessment on Monday.
- Partner selection sheet
Assignments
- Review and study for the Unit 2 assessment.

Tuesday, 14 November
In class
- Sun screen chemistry experiments.
Assignments
- Please complete the following survey by the end of the week with your parents.
- Partner assessment on atomic structure Monday, 20 November.

Friday, 10 November
In class
- Warm up exercises: Quantum mechanics
- Return and discuss your work on the Coulombic Interactions assessment.
- The Chemistry of Sun Protection.
- Why do We Have to Wear Sunscreen?
- Background reading and discussion.
- Form working groups and begin experimental design.
Assignments
- Dress for laboratory work on Tuesday.
- Bring the materials agreed upon by your working group.

Wednesday, 8 November
In class
- I’m sorry that I am not able to be with all of you today. My son is sick and I am taking care of him. Please work hard on the materials I have left with the substitute and treat them with the same respect and kindness you treat me. See you on Friday.
- Whiteboard problem set: quantum mechanics and electron configurations. Use the following resources as needed:
- cK-12 section 7.14, The de Broglie Wave Equation
- cK-12 section 7.15, Quantum Numbers
Assignments
- Read sections 7.18 and 7.19 in cK-12 before the start of class on Friday.
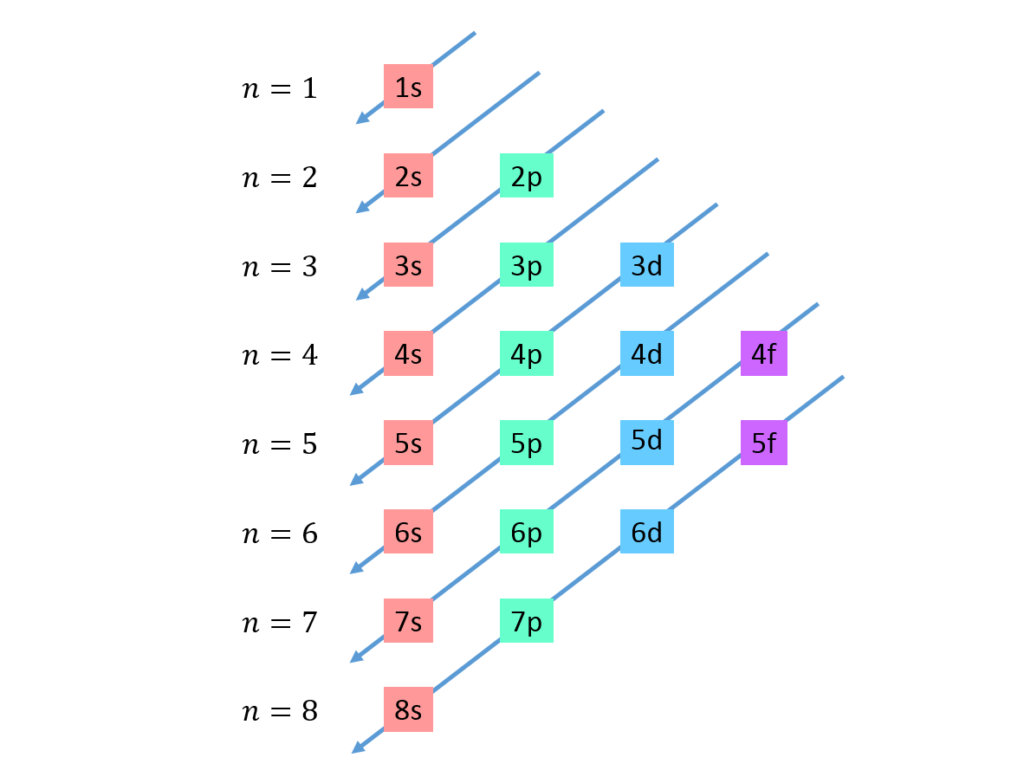
Monday, 6 November
In class
- Discuss your dart simulation plot.
- Q&A on the reading assignment.
- How are electrons arranged in atoms?
- Drawing practice: how to sketch atomic orbitals.
- A Better Way to Picture Atoms
- Electron configurations
Assignments
- Read sections 7.10 – 7.15 in cK-12 before class on Wednesday.
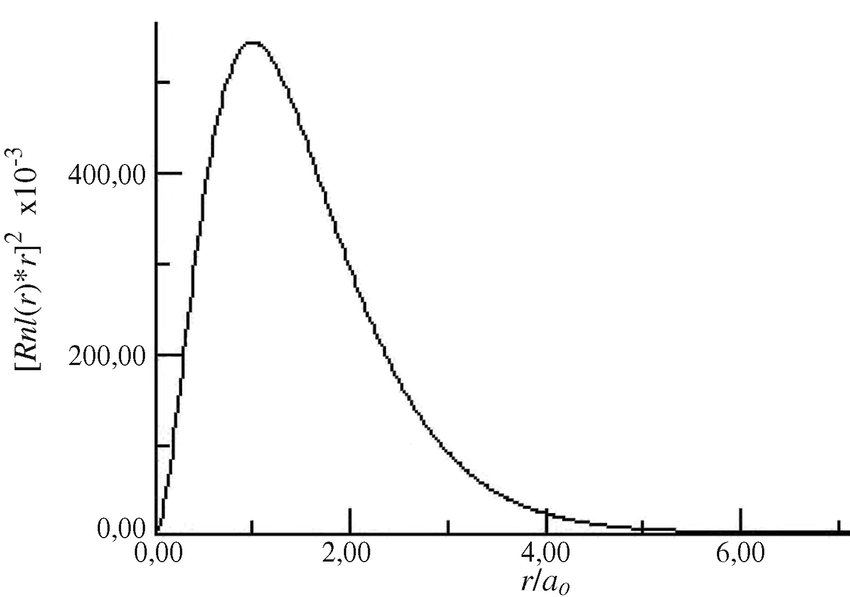
Thursday, 2 November
In class
- Short assessment: Coulombic Interactions.
- The atom as described by quantum mechanics.
- Is light a wave or a particle? (The Original Double Slit Experiment)
- Are electrons a wave or a particle? (The Quantum Experiment that Broke Reality)
- Modeling the behavior or the electron in a hydrogen atom with darts.
Assignments
- Read sections 7.7, 7.8, and 7.9 in cK-12 before the start of class on Monday.
- Print the graph you created in question 1 in the Analysis section of the simulation we conducted today in class. Tape this graph into your notebook.
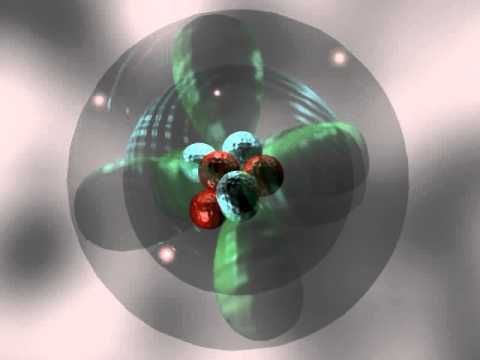
Tuesday, 31 October
In class
- Warm up exercise: Coulomb’s law concepts
- What is the electron in an atom of hydrogen doing to produce its spectrum?
- The Quantum Model of the Atom: Part 1
- What Really is a Quantum Jump?
Assignments
- Review for a short assessment on Coulomb’s law next class.
- TBA
Friday, 27 October
In class
- Warm up exercises: electromagnetic radiation.
- Measuring the wavelengths in the spectrum of hydrogen.
- What factors affect the force of attraction between charged particles?
- Coulomb’s Law PhET simulation
- Feedback (finally!) on the Unit 1 assessment and the isotopes quiz.

Wednesday, 25 October
In class
- Finish work on the case study.
- Whiteboard problem set: electromagnetic radiation calculations.
- Waves Intro PhET simulation
- Measuring the wavelengths in the spectrum of hydrogen.
Monday, 23 October
In class
- Isotopes short-form assessment.
- Continue work on the case study:
- Stage 1: What Would an Answer to this Question Look Like? (10 min)
- Stage 2: Examine the Data (15 min)
- Stage 3: Make a Detailed Plan (10 min)
- Stage 4: Crunch the Numbers (20 min)
- Stage 5: Reflect on the Calculations (5 min)
Assignments
- Complete sections 7.1 and 7.2 in cK-12 before the start of class on Wednesday.
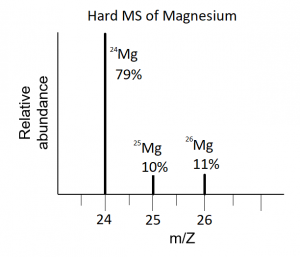
Thursday, 19 October
In class
- Discuss exercises 21, 23, and 25.
- Case Study: Is it a Meteorite?
Assignments
- Review the material on isotopes for a quiz on Monday. The Isotopes problem set (answer key now posted) given out last class is a good resource for your review. As always, you are allowed a note sheet.
- Finish the three questions on p. 2 of the case study before class if you didn’t do so in class today.

Tuesday, 17 October
In class
- Warm up: atomic structure.
- Discuss the alpha scattering experiment.
- Are all atoms of an element alike?
- Isotopes
Assignments
- Complete exercises 21, 23, and 25 from Chapter 2 in the Chemistry: Atoms First book before the start of next class. Section 2.3 may be good review if you need help answering these three problems.
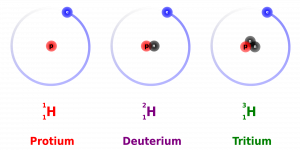
Friday, 13 October
In class
- How do we know about the structure of atoms?
- The History of Atomic Chemistry
- Build an Atom PhET simulation.
- Whiteboard problem set.
Assignments
- Complete the reading and practice exercises for section 6.12 in cK-12 before the start of next class.
Wednesday, 11 October
In class
- Unit 1 assessment.
Assignments
- Read section 6.1 and 6.4 – 6.8 in cK-12 before class on Friday.

Friday, 6 October
In class
- Atomos and the Greeks: Can something be indivisible?
- Just how small is an atom?
- TED-Ed video
- Length units of the atomic realm.
- Atomic spectrum of hydrogen
- Review for the Unit 1 exam.
Assignments
- Make your note sheet and review for the Unit 1 assessment next class.